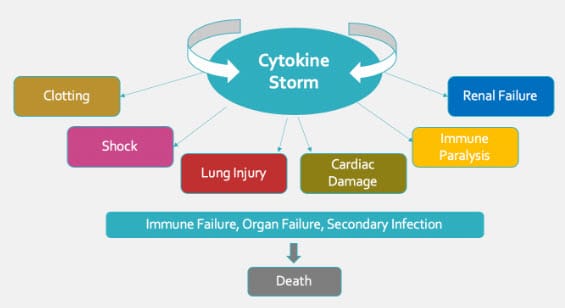
Both implantable and transcutaneous VNS has been extensively studied in animals, with more recent studies in humans confirming its safety and effectiveness. In rat models of sepsis, VNS attenuates the release of pro-inflammatory cytokines, prevents hypotension, and modulates coagulation and fibrinolysis, thus improving survival 23-25. This suggests that VNS has the therapeutic potential prevent hospitalized patients with COVID-19 from progressing to respiratory failure and death.
An in-depth review of the effectiveness of taVNS on the modulation of inflammation was recently published 12. The following information is taken directly from Current Directions in the Auricular Vagus Nerve Stimulation I – A Physiological Perspective (Front Neurosci. 2019;13:854. 2019) 12:
“Inflammation processes are governed through interrelated humoral and neural reflex pathways (Tracey, 2009; Miller and Raison, 2015). In particular, chronic inflammation is based on deregulation of metabolic and immune functions, whereas the imbalance between pro-inflammatory and anti-inflammatory cytokines seems to be decisive in disease progression (Neurath, 2014). Abnormal and chronic inflammation is implicated in, causes and advances, numerous wide-spread chronic diseases as diabetes mellitus and is, for example, a major hindering factor in effective neuroprotection in the brain, e.g., after stroke.
The vagus nerve (VN) provides a first-line defense against infection and inflammation in the periphery to restore homeostasis via conducting information to/from the brain to regulate the immune system. VN is a major component of the neuroendocrine-immune axis (Bonaz and Pellissier, 2016). For instance, even fever, as a brain-mediated response, is signaled to the brain via afferent VN responding to peripheral proinflammatory cytokines, in addition to blood-borne routes for the fever’s signaling (Hansen et al., 2001).
The parasympathetic outflow along VN, i.e., activation of the parasympathetic system, has only anti-inflammatory effects. This was shown by the inverse relationship between VN-mediated parasympathetic markers of the heart rate variability (HRV) and inflammatory markers (Thayer and Fischer, 2008). In contrast, the sympathetic nervous system may have both pro-inflammatory and anti-inflammatory effects.
In general, VN is involved in mainly three reflex pathways with a clear anti-inflammatory role:
- The anti-inflammatory hypothalamic-pituitary-adrenal axis. Here afferent VN fibers sense the level and location of injury/infection in that pro-inflammatory cytokines and/or endotoxins activate VN endings. Somatotopic maps in NTS become activated. Consequently, special neurons in hypothalamus activate the release of hormone adreno-corticotrophin by the hypophysis, stimulating the release of glucocorticoids by the adrenal glands to decrease peripheral inflammation.
- The anti-inflammatory vago-vagal reflex, known also as the cholinergic anti-inflammatory pathway (Borovikova et al., 2000; Tracey, 2007). Here infection-activated afferent VN fibers synapse with and generate an outflow along efferent VN fibers releasing acetylcholine at their synaptic endings. The acetylcholine binds to surface receptors of macrophages and suppresses the production and release of pro-inflammatory cytokines by these macrophages. Interestingly, the tonic neural activity of this cholinergic anti-inflammatory pathway is essential because, when it is impaired, over-inflammation results with an unrestrained cytokine release damaging tissue (Mercante et al., 2018a). From this perspective, vagus nerve stimulation (VNS) enhances the activity of immune-related neural circuits and confers protection of the human body.
- The splenic sympathetic anti-inflammatory pathway. Here the infection-activated afferent VN yields outflow along the efferent VN which stimulates the adrenergic sympathetic nerve in the spleen, releasing norepinephrine at its endings. Then norepinephrine binds to splenic lymphocytes and leads to acetylcholine release by lymphocytes, whereas acetylcholine, in turn, inhibits the release of pro-inflammatory cytokines by splenic macrophages.
Thus, the innate immune system is subjected to a closed-loop reflex modulation via afferent VN fibers, as illustrated in Figure 2A, whereas the activity of the efferent VN maintains homeostasis by limiting pro-inflammatory responses and avoiding immunosuppression. For instance, the role of VN is proposed in informing the brain about peripheral inflammation related to coronary artery disease and in actively modulating the disease related inflammation (Gidron et al., 2006).
An artificial VNS has been shown to harness this natural reflex (Figure 2C). The modulation of VN results in decreased pro-inflammatory and increased anti-inflammatory cytokines, which is effective in suppression of over-inflammation, prevention of tissue injury, and improved survival. For instance, aVNS reduced pro-inflammatory cytokines, as shown in a clinical trial (Stavrakis et al., 2015), and increased norepinephrine levels, as reviewed in Beekwilder and Beems (2010), which supports anti-inflammatory aVNS effects.
In particular, VNS was shown to rebalance the working point of autonomic regulation of the immune system into a protective range avoiding pro-inflammatory responses and, on the other hand, avoiding immunosuppression (Tracey, 2009). Here the working point is defined as the magnitude of innate immune responses relative to the infection or injury stimulus. Chronic changes can unfavorably increase or decrease the working point with the resulting overshooting immune response (with tissue damage, sepsis, or even death) or immunosuppression (with secondary infections), respectively.
In animals, VNS had favorable effects on rheumatoid arthritis in rats (Koopman et al., 2017). VNS reduced surgery-induced intestinal inflammation and improved postoperative intestinal transit in mice, supporting the anti-inflammatory effect of VNS (Matteoli et al., 2013). In addition, VNS prevented the development of shock in rats through inhibited synthesis of the tumor necrosis factor (cytokines) (Borovikova et al., 2000). aVNS was shown to be efficient in mice with lethal endotoxemia or polymicrobial sepsis while reducing systemic tumor necrosis factor due to anti-inflammatory aVNS effects (Huston et al., 2007). aVNS (auricular vagus nerve stimulation) suppressed lipopolysaccharide-induced inflammatory responses in endotoxemic rats through reduced pro-inflammatory cytokines, indicating that aVNS modulates the immune function through the cholinergic anti-inflammatory pathway (Zhao et al., 2012).
In humans, potential therapeutic applications of aVNS are related to chronic inflammatory conditions. These are rheumatoid arthritis, see a clinical trial in Becker (2007), inflammatory bowel disease (Crohn’s disease, ulcerative colitis), and postoperative ileus in order to restore intestinal homeostasis, as reviewed in Tracey (2007), Marshall et al. (2015), and Bonaz and Pellissier (2016).”