Medical Disclaimer: The information presented on this website is provided as an information resource only, and is not to be used or relied on for any diagnostic or treatment purposes. This information is not intended to be patient education, and does not create any patient-physician relationship.
VNS Trial for COVID-19 Pneumonia
Brief Study Description
The objective of this study is to determine the therapeutic effect and tolerance of transcutaneous vagus nerve stimulation in patients with moderate, severe or critical pneumonia associated with Coronavirus Disease 2019 (COVID-19).
The vagus nerve can be electrically stimulated with implantable or transcutaneous methods which significantly reduce of pro-inflammatory cytokines and improve clinical outcome in a variety of medical conditions including inflammatory bowel disease, rheumatoid arthritis and sepsis.
COVID-19 patients appear to have an excessive immunological response and ground-glass chest x-ray findings indicative of inflammatory damage to the lungs. Transcutaneous vagus nerve stimulation suppresses excessive inflammatory reactions and may be useful in controlling the “cytokine storm” that is responsible for ARDS (Acute Respiratory Distress Syndrome) and pulmonary failure observed in COVID-19.
The study will evaluate of 5 minutes of transcutaneous auricular vagus nerve stimulation (taVNS) delivered four times daily to hospitalized COVID-19 patients.
taVNS will be administered to consenting adult patients hospitalized with COVID-19 and diagnosed with moderate or severe pneumonia requiring supplemental oxygen. All study subjects will continue to also receive any treatment considered standard of care.
Study Results
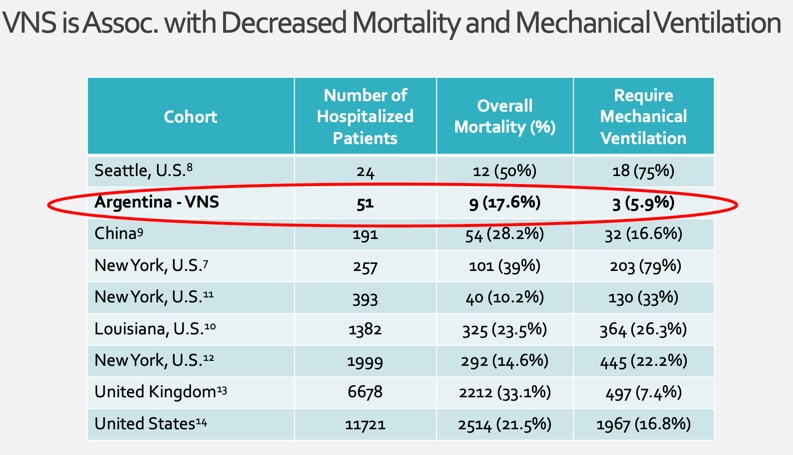
Conducted in Buenos Aires, the study demonstrated that using transcutaneous vagus nerve stimulation (taVNS) used 4 times daily in hospitalized patients with COVID-19 was associated with a low requirement for mechanical ventilation and low mortality compared to other hospitalization cohorts.
Transcutaneous vagus nerve stimulation is a potent inhibitor of inflammatory cytokines. Ideal for any hospital setting, taVNS is low cost, safe and a single device can be used among different patients simultaneously